 |
|
|
|
|

Senior Communication and Press Officer

No single State, organisation, company, or community can meet malaria's challenges alone. Cooperation and coordination are important elements to ensure an effective and rapid response to control malaria. In line with DNDi's goals to meet the needs of people most threatened by neglected diseases, two antimalarial drugs have been delivered within the multi-partner Fixed-Dose, Artesunate-Based Combination Therapies (FACT) Project, which was created in 2002 under the umbrella of MSF and then transferred to DNDi in 2003 in coordination with the UNICEF-UNDP-World Bank-WHO Special Programme for Research and Training in Tropical Diseases (TDR).
As Executive Director of DNDi, Dr. Bernard Pécoul offers his take on how partnership is so valuable in addressing urgent needs for those affected by malaria.
Could you give us some background on the FACT Project?
The FACT Project responds to specific needs in identifying suitable development candidates for combinations of existing antimalarial drugs to control malaria resistance. It emerged that co-formulation therapies derived from artemisinin could be the right answer. This was clearly outlined by WHO and international NGOs like MSF, especially given the complexity to develop a national control programme and pertinent strategies to fight malaria. With such co-formulations, treatment compliance and efficient implementation were important prerequisites for the project.
What is the objective of the FACT Project?
The purpose of the FACT Project and the partnership was to clearly develop products as public goods which will be available and affordable to the most affected population.
The new product should be adapted to field needs, offering a simple regimen of 1 to 2 tablets administered once a day, adapted to paediatric use, presented in practical packaging, and providing a stable product under tropical conditions.
Who are your partners in this project?
The FACT Project is a perfect illustration of DNDi's collaborative model with different categories of partners from around the world: France's Bordeaux University and Ellipse Pharma, along with Brazil's public pharmaceutical company Farmanguinhos, were responsible for formulating and producing the fixed-dose combinations, the University of Sains Malaysia was responsable for analytical and bioanalytical studies, and pharmacokinetics and Thailand's Mahidol University, and Shoklo Malaria Research Unit, along with Burkina Faso's Centre for Malaria Research, the Kenya Medical Research Institute, and National Institute of Malaria Research (NIMR) in India, carried out clinical trials in-country, with scientific support from the UK's University of Oxford. Sanofi-aventis and Cipla are two partners from the private pharmaceutical sectors that have contributed or will contribute, as is the case for Cipla, in the production of drugs and in their availability for the endemic countries.
A close working relationship like the one DNDi has developed with Brazilian regulatory authorities should also take place and be managed in order to facilitate the drugs' implementation in each endemic country. By pooling the expertise and resources of research institutes and drug developers and producers, regulatory authorities, national malaria control programmes, and NGOs all over the world, DNDi can lead valuable drug combination candidates all the way to becoming treatments for the patients.

Children swim in a pond in Acre, Legal Amazon, Brazil. Pools are breeding grounds for malarial mosquitoes.
Acre has been the focus of National Malaria Health authorities, where ASMQ, an antimalarial drug developed by Farmanguinhos/Fiocruz and DNDi, has shown to be field-effective for 17,000 patients.
Who is funding this project?
Throughout the lifespan of the project, public financing has been critical in allowing the project to meet its primary objective of developing two effective antimalarial medicines, ASAQ and ASMQ.
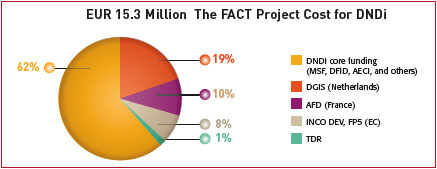
The FACT Project budget of EUR 15.3 million has been supplied by public funds, including project-specific funding provided by the European Union (Framework Partnership 5; FP5), the French Development Agency (Agence Française de Développement; AFD), and the Dutch Ministry of Foreign Affairs (DGIS).
In addition, core organisational funding from MSF International, the Spanish Agency for International Cooperation (Spanish Agencia Española de Cooperación Internacional; AECI), and the UK Department for International Development (DFID) has been used by DNDi in order to make the FACT products available.
Valuable in-kind contributions have supplemented the total budgetary costs shown; in particular, the in-kind contributions of Farmanguinhos/Fiocruz, sanofi-aventis and TDR were significant throughout the entire process of FACT Project development as were the in-kind contributions of individual experts. We may even go further in saying that these in-kind contributions were equivalent to the FACT development cost for DNDi (see box).
With partners from all over the world, how do you manage to carry out such Project?
I think that all the FACT partners understand the FACT rationale and objectives and remain committed to them. We also have to highlight the key role played by Jean-René Kiechel, our Senior Project Manager, who, with his long experience, competence and far-sightedness, manages to coordinate efforts and create a viable environment for the FACT Project to move forward in a constructive way.
As captain of the boat, Jean-René also ensures a thorough follow-up of the different phases of the project's development, organising numerous ad hoc as well as regular biannual meetings with committed and motivated partners to assess project progress and results, analyse critical issues, agree on solutions to issues, and review common strategies and redistribute tasks when necessary.
The FACT Project also proves its worth in bringing in an independent panel of experts on malaria who meet on a regular basis to contribute with advice and critical guidance. We can mention here the important contributions of Nick White, François Nosten and Piero Olliaro, among many others to whom we are greatly indebted.
And what about the limitations of the exercise, do you face any hurdles with the FACT Consortium?
Since the partnership involves actors from Africa, Latin America, South East Asia and Europe, we have to find ways of maximising collaborative efforts within the background of cultural differences. This also applies in getting consensus from the public and private sectors concerned. Of course, as soon as we face a problem at one stage of the project, it has already had consequences on the process as a whole.
For instance, if we look at the mefloquine supply for the artesunate-mefloquine (ASMQ) project, when the source of mefloquine from Abbott was cancelled, the project was seriously held up. As a consequence, it took us 12 months to identify and validate the tablet production process of another supplier of good quality mefloquine. Such delays were out of our own control but nevertheless had impact on the progress of the project.
A different problem also occurred with artesunate-amodiaquine (ASAQ) project, in the pivotal clinical trial phase that took place in Burkina Faso. The recruitment of patients was due to take place over a period of high transmission of malaria. As the high transmission malaria season progressed without the team being able to include all required patients, it became necessary to pursue the study the following season, six months later. We were thus unable to obtain timely results for the first season as planned.
What are the challenges laid ahead?
Firstly, and what represent the most important challenge for us, is indeed to render the products available to patients: substitute co-blisters by the co-formulation; secondly, to extend the use of these drugs in other geographic areas; thirdly, to make others understand that we are not of a competitive spirit. We aim at rendering ACT therapies widely available to control malaria. Several drug combinations will be required to achieve such an objective in a context which is extremely different from country to country and to address a disease affecting 350-500 million patients per year. As a fourth challenge, we require active participation at the implementation stage from a different group of partners in order to monitor the safety, efficacy (monitoring of resistance), and effectiveness of the products in field conditions. And finally, for the next generation along with Medecines for Malaria Venture (MMV), we should promote the search for new malaria drugs in order to avoid being confronted with the situation encountered few years ago, the development of resistance to existing drugs.
As a conclusion I would also like to emphasise that the FACT Projects were a kind of test for DNDi to assess the Partnership model as well as DNDi's own capabilities to successfully perform research and development. The two antimalarial products are satisfying proofs of these efforts. And now they are model to follow for the development of improved treatments in the field of trypanosomiasis and visceral leishmaniasis diseases.