
GARDP
Together with WHO, DND

Delivering antibiotics for all
Dr Manica Balasegaram, Director of GARDP, explains how drug-resistant superbugs threaten progress reducing child mortality, and GARDP’s objectives to develop new antibiotics.
WATCH FILM
Key Achievements
Developed a business plan that prioritizes R&D strategies on global health priorities
Secured EUR 56 million in additional funding
Published a scientific roadmap and target product profiles for sexually-transmitted infections
Partnered with Entasis Therapeutics to develop a novel, first-in-class antibiotic for drug-resistant gonorrhoea
Conducted a survey on antibiotics used to treat late-onset sepsis in newborns, which indicates high levels of drug resistance in some settings
Published a strategy to address antibacterial resistance in newborn babies
Reviewed some 20 assets from several pharmaceutical companies and launched REVIVE, an online resource for the antimicrobial R&D community
Established a country partnership with South Africa
Opened a joint DNDi/GARDP office in South Africa
Built a skilled team with expertise from a range of sectors and scientific networks
R&D programmes
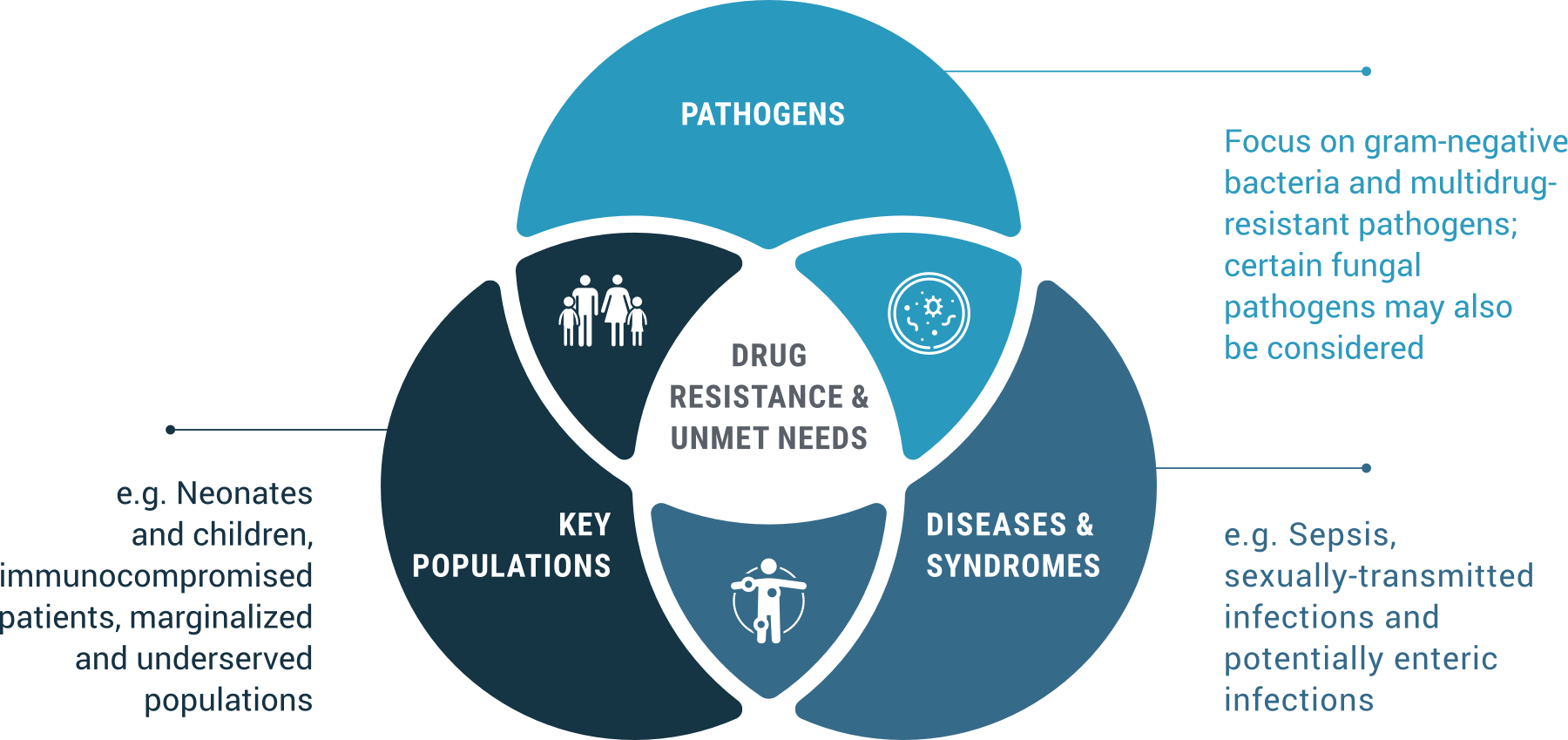
GARDP’s priorities are determined by considering the intersection between: WHO priority pathogens; specific populations’ health needs; and individual diseases and broader syndromes. In addition to a paediatric platform to be launched in 2018 on paediatric antibiotics, GARDP’s first Business Plan focuses on the following programmes of work:
Neonatal sepsis
Every year, around 214,000 deaths in newborns are attributable to drug-resistant infections, which represent a major barrier to achieving the Sustainable Development Goal to reduce child mortality. A considerable challenge is the lack of evidence about appropriate treatment of drug-resistant infections in newborns. GARDP hopes to develop this evidence base. An initial feasibility survey completed in 2017 shows high levels of drug resistance in neonatal units. GARDP will work on new and improved treatments for newborns, through partnerships including with St George’s, University of London and the Paediatric European Network for Treatment of AIDS (PENTA) Foundation. A study on the pharmacokinetics of a potential drug candidate and a global observational study have been approved. Both are ready for implementation in 2018.
Sexually-transmitted infections
With around 78 million new cases each year, gonorrhoea is one of the most common sexually-transmitted infections. Development of resistance is a major concern. In 2017, GARDP entered its first partnership agreement with Entasis Therapeutics on the development of zoliflodacin – a novel, first-in-class oral antibiotic that has high activity against drug-resistant gonorrhoea. In parallel to sponsoring a phase III clinical trial, including in the EU, South Africa, Thailand, and the US, GARDP will carry out non-clinical activities to ensure zoliflodacin is effective against recent and geographically diverse strains of gonorrhoea. If zoliflodacin receives regulatory approval, Entasis will grant GARDP a licence in 168 low- and middle-income countries. GARDP is committed to affordable and equitable pricing.
Antimicrobial memory recovery and exploratory
This programme focuses on recovering knowledge, data, and assets of forgotten, abandoned or withdrawn antibiotics, and to identify new treatments. A memory recovery workstream is evaluating recovered molecules for their potential value for antimicrobial drug development. In 2017, some 20 assets were reviewed from several pharmaceutical companies. GARDP has developed REVIVE, a digital space to facilitate learning and sharing good practice in the conduct of antimicrobial drug R&D. So far, more than 100 experts have engaged with REVIVE. A programme of webinars and workshops is planned for 2018. The discovery and exploratory workstream supports pre-clinical research. This includes building a long-term portfolio of therapeutic interventions to address the ultimate and unavoidable development of resistance to any new therapy that is brought to patients.

