 | |

Nifurtimox-eflornithine: a simpler, safer, and more effective combination for stage 2 HAT patients
Els Torreele
Project Manager DNDi
Project Manager DNDi
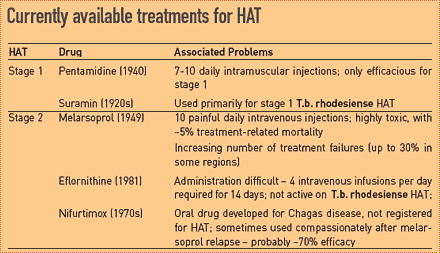
Each of the few drugs available for people suffering from stage 2 HAT has problems of toxicity, efficacy, and complicated use, and/or the risk of developing resistance. It will take at least another 5-7 years before any new drug candidate for stage 2 HAT reaches patients. Meanwhile, drug combinations are considered the best way to meet some of the patients’ needs in the short to medium term. Ideally, drug combinations allow for reductions in drug dosage, especially important in the case of toxic drugs, reduced treatment duration, and ease of use, and also protect against resistance development.
Over the past years, a few clinical studies were carried out in the use of combinations with melarsoprol, eflornithine, and/or nifurtimox (mainly by the Institute of Tropical Medicine Antwerp (ITMA) in Democratic Republic of Congo (DRC) and MSF/Epicentre in Uganda and Congo-Brazzaville). These studies indicated that nifurtimox-eflornithine was a promising option, but more research was needed to identify a suitable dose-regimen and document its safety and efficacy.
Beginnings of the NECT trial (nifurtimox-eflornithine combination): an Epicentre initiative
During 2002-03, Epicentre and MSF-Holland (MSF-H) developed a randomized controlled study, the NECT study, comparing a simplified combination of elflornithine (7 days, 2 infusions/day) plus 10 days of oral nifurtimox with the standard elflornithine treatment (14 days, 4 infusions/day). The study, designed as a non-inferiority study with a minimum of 140 patients in each arm to achieve statistical significance, started in August 2003 in an MSF-H HAT treatment project in Nkayi, Congo- Brazzaville.
By the end of 2004, a total of 103 patients had been enrolled in the study (of the 280 needed). Thanks to the intensive screening and treatment activities of MSF-H in the Nkayi region, the HAT incidence had dropped to very low levels, and it was decided to stop further patient recruitment at that site. As prescribed in the study protocol, all enrolled patients were followed up for 18 months after the treatment to evaluate efficacy.
DNDi comes to the support of NECT
In April 2004, MSF, Epicentre, WHO, TDR, STI, DNDi and independent experts met to review the available clinical evidence on the use of nifurtimox in HAT. The group was informed of the on-going MSF/Epicentre nifurtimox-eflornithine trial in Nkayi, Congo-Brazzaville. While initial results on safety and efficacy looked very promising, the group was told that the study could not be completed due to exhaustion of the patient pool in the study area.

Considering the expected benefit to patients if this simplified combination regime worked, and the huge effort already made to enroll over 100 patients, it was decided that the best way forward would be to work together to strengthen and expand the trial and ensure sufficient enrolment for statistically relevant results. DNDi committed to sponsor and manage three sites in DRC (Isangi, Dipumba, Katanda) and TDR committed to sponsor two sites in Uganda (Omugo, Moyo). New implementation partners included the national HAT control programmes of DRC and Uganda, and the Swiss Tropical Institute. Epicentre continues to ensure the medical coordination, and will also be responsible for data management and analysis. The goal of the multi-centre multi-partner effort is to garner sufficient evidence on the clinical safety and efficacy of the simplified combination scheme to obtain a WHO-recommendation for use.

DNDi sponsors three new study sites in DRC
In 2005, an amendment to the original Epicentre study protocol was finalized to integrate new study sites and international guidelines for Good Clinical Practices. Together with the national HAT control programme (PNLTHA) in DRC, DNDi made several site visits to assess and identify possible study sites. These visits included an assessment of what was needed to build clinical research capacities. The first selected site was Isangi oriental province of DRC, where MSF-B had started a HAT screening and treatment activity at the local hospital. After refurbishment of the clinical and laboratory facilities and study-specific training of the staff, MSF-B and Epicentre started implementing the NECT study in Isangi in July 2005. In 11 months, 64 patients were enrolled at this site.
Meanwhile, two additional study sites were selected, the Dipumba hospital in Mbuji Mayi and the HAT treatment center of Katanda, ~70 km away, where, after a similar round of rehabilitation, re-equipping and training, the first patients were enrolled in April and May 2006 respectively. At these sites, the Swiss Tropical Institute is working in close association with the PNLTHA teams to implement the study, with Epicentre as medical coordinator.
Current status of the Phase III clinical trials
By September 2006, the three DNDi-supported sites in DRC had enrolled around 160 patients to complement the 103 enrolled by MSF/Epicentre in Nkayi. Recruitment of the full cohort of 280 patients is expected to be completed by the end of 2006. Taking into account the 18 months follow-up period, final data should be available by mid 2008.
Meanwhile, preliminary analysis of the safety and efficacy data from the first 103 patients in Nkayi (follow-up almost completed) are very promising, and suggest that the safety and efficacy of NECT is not inferior to the standard eflornithine treatment. If the final results of the full study cohort confirm this trend, this combination should represent a better alternative over current first-line treatment options for stage 2 HAT - a less toxic, more efficacious cure than melarsoprol (see The Fatal Sleep), and easier to use than eflornithine. A WHO-recommendation in support of the use of nifurtimox-eflornithine as first line treatment for stage 2 HAT would then pave the way to implementation and policy change by HAT-endemic countries.
Published by Drugs for Neglected Diseases Initiative - 1 Place St Gervais 1201 Geneva Switzerland - Photo credits: DNDi
Editor: Ann-Marie Sevcsik - Tel: +41 22 906 9230 - Fax: +41 22 906 9231 - www.dndi.org
Editor: Ann-Marie Sevcsik - Tel: +41 22 906 9230 - Fax: +41 22 906 9231 - www.dndi.org