 |
|
 An Improved Treatment for Sleeping Sickness
By Gerardo Priotto, Principal Investigator, Epicentre and Ann-Marie Sevcsik, DNDi’s Scientific Communications Manager |
The NECT Phase III pivotal study conclusively demonstrates that the coadministration of oral nifurtimox and intravenous eflornithine (NECT) is a safe, effective treatment for stage 2 HAT patients, and more practical than eflornithine monotherapy.
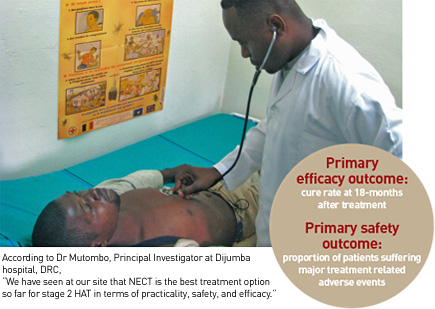 Background: the need for combination therapies for stage 2 HAT The rationale for the NECT (nifurtimoxeflornithine combination therapy) study lies in the fact that, for stage 2 HAT, there were few available drugs, each with serious limitations (see existing treatments), and not even one new drug in clinical development. With the third major HAT epidemic of the twentieth century devastating rural communities in sub-Saharan Africa – particularly in Angola, the Democratic Republic of the Congo (DRC), southern Sudan, and northwestern Uganda – from the late 1980s through the late 1990s, many health practitioners felt an urgency to do something to improve patient care. The toxic side effects of melarsoprol, its declining efficacy, and the lack of realistic alternatives prompted the search for a viable better alternative which could drastically reduce the use of this very toxic drug becoming ineffective. NECT emerges as most promising of combination therapies for stage 2 HAT
The Institute of Tropical Medicine (ITMA) in the DRC, in addition to Médecins Sans Frontières (MSF) and Epicentre in Uganda, carried out much of the early work examining combinations. In two clinical studies in Uganda looking at combinations of melarsoprol, eflornithine, and/or nifurtimox, the combination of nifurtimox and eflornithine was shown to be the most promising option (1),(2), but more research was needed to identify a suitable dose regimen and to confirm its safety and efficacy.  Epicentre and MSF undertake the NECT study in Congo-Brazzaville Epicentre and MSF undertake the NECT study in Congo-BrazzavilleEncouraged by these early results, in 2003 a team from Epicentre and MSF began a randomised, controlled trial (RCT) (3) in Nkayi, the Republic of the Congo. A simplified schedule of 7-days eflornithine in combination with 10-days nifurtimox (NECT) was compared with the standard, 14-day eflornithine schedule. With the aim to enrol a minimum of 140 patients for each treatment option to statistically determine noninferiority, the primary objectives were to evaluate the efficacy and safety of NECT in comparison with eflornithine monotherapy. Unfortunately, enrolment could not be completed at the Nkayi site due to the drastic reduction of HAT incidence related to the intensive screening and treatment activities of MSF in the region. By the end of 2004, a total of 103 patients (of the 280 needed) had been enrolled in the study. DNDi joins in support of NECT In April 2004, a group of experts including representatives from Epicentre, Médecins Sans Frontières (MSF), Swiss Tropical Institute (STI),TDR (the UNICEF/UNDP/World Bank, and WHO Special Programme for Research and Training in Tropical Diseases), World Health Organization (WHO), and DNDi met to review the available clinical evidence on the use of nifurtimox for HAT. The group was informed of the ongoing NECT study and its promising initial results. Considering the expected benefit to patients if this simplified combination regime worked, and the huge effort already made to enrol over 100 patients, it was decided that the best way forward would be to work together to strengthen and expand the trial and ensure sufficient enrolment for statistically relevant results. At the meeting, DNDi committed to sponsor three sites in the Democratic Republic of the Congo (DRC). In addition to Epicentre and MSF who implemented and managed the study in Isangi, new implementation partners included the national HAT control programme of DRC and the STI. Epicentre continued to ensure the medical coordination, data management, and analysis for the full study. Epicentre also worked with all partners to prepare a protocol amendment to extend the study into a multicentre, multicountry setting. Implementing three new study sites in DRC Together with the national HAT control programme (PNLTHA) in DRC, DNDi and Epicentre made several trips to assess and identify possible study sites in Isangi, Dipumba, and Katanda. These visits included an assessment of what was needed to conduct the trial to international Good Clinical Practice (GCP) research standards and to strengthen overall clinical research capacities. The Isangi site was first chosen in the Oriental province of DRC, where MSF had started a HAT screening and treatment activity at the local hospital. After refurbishment of the clinical and laboratory facilities and study-specific training of the staff, MSF and Epicentre started implementing the study in July 2005. In eleven months, 64 patients were enrolled at this site. Meanwhile, two additional study sites were selected, the Dipumba hospital in Mbuji Mayi and the HAT treatment centre of Katanda, about 70 kilometres away. After a similar round of rehabilitation, equipment and training, the first patients were enrolled in April and May of 2006, respectively. At these sites, the Swiss Tropical Institute (STI) worked in close association with the PNLTHA teams to implement the study, with Epicentre as the medical coordinator. The three DNDi -sponsored sites were regularly monitored by STI. 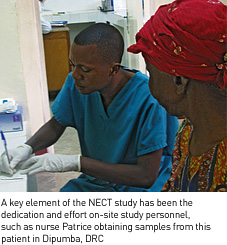 NECT is shown to be safe and efficacious in a randomised, controlled, GCP-compliant trial with excellent follow-up NECT is shown to be safe and efficacious in a randomised, controlled, GCP-compliant trial with excellent follow-upBy November 2006, the targeted enrolment of 280 patients had been reached, with the three DNDi -supported sites in DRC enrolling 184 patients to complement the 103 enrolled by MSF/Epicentre in Nkayi. Taking into account the 18 month follow-up period for the primary efficacy measure, final data analysis was completed in September 2008. The final results demonstrate the noninferiority of NECT for efficacy, and a favourable safety profile. With an excellent patient follow-up at 18 months after treatment of 93%, cure rate was shown to be comparable between the two treatment arms, and NECT showed a significant advantage in a number of secondary indicators. In terms of safety, both treatments were well tolerated and had low fatality rates. These data confirm that NECT represents an improved alternative over current first-line treatment options for stage 2 HAT - a less toxic, more efficacious cure than melarsoprol, and easier to use than eflornithine monotherapy. A submission for inclusion of nifurtimox, to be used in combination with eflornithine in the WHO Essential Medicines List 2009 is being prepared. Final study results are being presented at a number of international meetings (including the 17th International Congress on Tropical Diseases and Malaria (ICTDM), held early October 2008 in Jeju, South Korea, the HAT Platform meeting, mid November 2008 in Brazzaville, Republic of the Congo, and the 57th Annual Meeting of the American Society of Tropical Medicine and Hygiene (ASTMH) which will be held in New Orleans, USA, 7-11 December 2008) and will be submitted for publication early 2009. 1 - Priotto G, Fogg C, Balasegaram M, et al. Three drug combinations for late-stage Trypanosoma brucei gambiense sleeping sickness: a randomized clinical trial in Uganda. PLoS Clin Trials. 2006;1(8):e39. 2 - Checchi F, Piola P, Ayikoru H, Thomas F, Legros D, Priotto G. Nifurtimox plus Eflornithine for Late-Stage Sleeping Sickness in Uganda: A Case Series. PLoS Negl Trop Dis. 2007;1(2):e64. 3 - Priotto G, Kasparian S, Ngouama D, et al. Nifurtimoxeflornithine combination therapy for second-stage Trypanosoma brucei gambiense sleeping sickness: a randomized clinical trial in Congo. Clin Infect Dis. Dec 1 2007;45(11):1435-1442. |
Published by Drugs for Neglected Diseases Initiative - 15 Chemin Louis-Dunant 1202 Geneva Switzerland - Photo credits: DNDi unless otherwise stated - Editor: Sadia Kaenzig - Tel: +41 22 906 9230 - Fax: +41 22 906 9231 - www.DNDi.org