 |
|
|
|
|

What is leishmaniasis?
Leishmaniasis is a poverty-associated disease with several different forms, of which the following two are most common:
• VL – fatal without treatment
• Cutaneous Leishmaniasis (CL) – has a spectrum of presentations, typically with self-healing or chronic lesions on the skin. VL is the primary disease target for DNDi, whereas CL is secondary, mainly because it is generally not a life-threatening disease.
What is the annual impact of leishmaniasis?
500,000 cases of VL;
1.5 million cases of CL (1)
51,000 deaths(2) 2,357,000 DALYs (2) (3)
Lack of surveillance systems and frequency of misdiagnosis means that it is difficult to estimate the true incidence and case-fatality rate of VL (1).
Where does leishmaniasis occur?
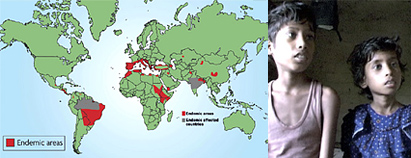
Leishmaniasis infects approximately 12 million people in 88 countries. VL affects poor, remote populations in 70 countries across Asia, East Africa, South America, and the Mediterranean region (see map). More than 90% of new cases are reported in the six most affected countries – Bangladesh, Brazil, India, Ethiopia, Kenya, and Sudan.
How is leishmaniasis transmitted?
Leishmaniasis is diverse and complex: more than 20 species of the kinetoplastid protozoan parasite Leishmania are transmitted to humans by ~30 species of phlebotomines sandflies.
What are the symptoms of VL?
VL is characterised by prolonged fever, enlarged spleen and liver, substantial weight loss, and progressive anaemia. These symptoms occur progressively over a period of weeks or even months. Coinfection with other infectious diseases is an increasing concern: HIV-VL coinfection has been reported in 35 countries worldwide. Almost all clinically symptomatic patients die within months if untreated.
What are the current treatments and their limitations?
The number of treatments has increased in the past decade, but there are numerous drawbacks specific to each type, such as difficulty to administer, long treatment time, toxicity, cost, and increasing parasitic resistance to treatment:
- Pentavalent antimonials: toxic and increasingly ineffective due to resistance; 30-day, hospital- based parenteral treatment
- Amphotericin B: dose-limiting toxicity; 15-20 day, hospital-based IV treatment
- Liposomal amphotericin B (AmBisome®): excellent, but expensive, IV infusions. Cold chain required
- Paromomycin: a low-cost parenteral (intramuscular) formulation of paromomycin (aminosidine) administered over 21 days, registered by Institute for OneWorld Health in India and now in phase IV, registered in India, registered for VL in the USA and Europe with remarkable activity but efficacy in Africa not yet determined
- Miltefosine: oral miltefosine registered in India in 2002, now being used in monotherapy and completing a Phase II trial of ‘miltefosine + AmBisome®’ sponsored by TDR, administered over 28 days, but expensive(4) and potentially teratogenic for humans.
Patients need a treatment which is oral, safe, effective, low cost, and short course (<10-day course).
(1) Desjeux P. Comp Immunol Microbiol Infect Dis. 2004; 27:305.
(2) WHO. Report of the 5th Consultative Meeting on Leishmania/HIV Coinfection. Geneva; 2007.
(3) WHO. TheWorld Health Report. Geneva; 2004. Available from www.who.int/whr/2004. Accessed Aug 12, 2008. DALYs are a measure of societal impact, being the sum of years of potential life lost due to premature mortality and the years of productive life lost due to disability.
(4) Through the WHO, significant cost reduction of both AmBisome® and miltefosine is available for the public sector of developing countries as of 2007.